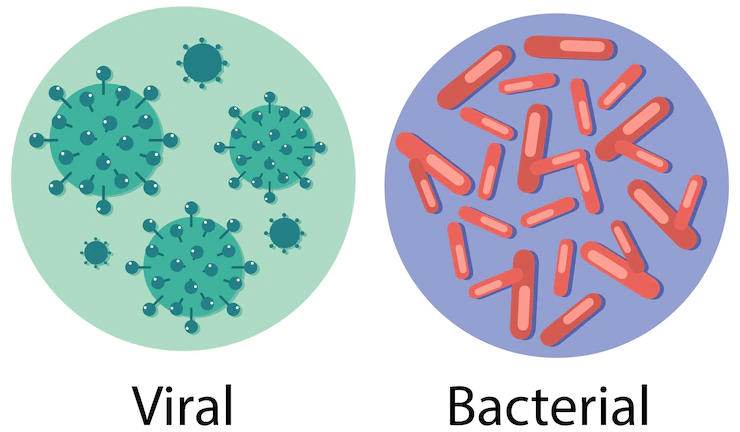
If you are looking for an enzyme that degrades starch, then you should know the difference between fungal and bacterial amylase.
Both enzymes function to break down starch and inhibit its recrystallization.
Bacterial amylases are more effective than those from fungi.
a-Amylase
The purified enzymes exhibit a pH range of 6.5 and an optimum temperature of 60degC. Outside these ranges, the enzyme loses activity. It has minimal activity at 35-40degC and no activity at 2.5-3.5 pH. However, at 60degC and a pH of 6.5, the enzyme retains 50% activity.
A-amylase is obtained from Bacillus licheniformis, which has a trading name of “Termamyl”. Microbial a-amylases are generally used in industrial processes and detergent applications, but they are also being studied for their potential as bioethanol biocatalysts. Hyperthermophilic amylases offer even greater bioethanol production capabilities.
The TAKA-amylase has a characteristic a-amylase fold and contains an insertion domain with a calcium-binding site. This enzyme is similar to a bacterial amylase but contains a distinct fold. Its b/a8-barrel insertion domain and C-terminal b-sandwich domain were studied using inhibitors to identify the active site.
The crude extract of -amylase contains approximately 59 kDa. Amylase expression increased with cell density. Protein synthesis is largely performed in the log phase, a stage where cellular constituents are synthesized at a constant rate.
Bacillus amyloliquefaciens a-amylase predominantly catalyzes the endohydrolysis of wheat starch. In addition, it may act as an a-exo-amylase. This enzyme changes the starch structure and results in an increased release of maltose and glucose.
A-amylase increases the rate of fermentation by providing additional substrates for yeast cells. Additionally, it extends the time of productive fermentation by shifting the yeast metabolism.
Despite its similarity in molecular mass, the a-amylase from sorghum grain is insensitive to high salt concentrations, making it suitable for use in starch conversion processes. Foods often contain high concentrations of salts to improve flavor and stabilize them.
a-Amylase is a starch-degrading enzyme
Amylase is a family of enzymes that hydrolyze starch to oligosaccharides. The enzyme a-amylase degrades starch by hydrolyzing its 1,4-a-glucoside bonds, while b-amylase degrades starch to release b-maltose.
Amylase activity is measured by the change in the color of starch in the presence of iodine, which is a substrate of the enzyme. Its activity is quantified by two different kinetic methods, using the enzyme nicotinamide adenine dinucleotide and iodine.
AmyPDSBD is a member of the a-amylase family, and its B domain is one of the smallest. This elongated architecture restricts substrate access, and it forms a structural lid above the active site. Moreover, AmyPDSBD has an extended cleft that points away from the catalytic site, and it is possible that this cleft is necessary to promote the degradation of raw starch granules.
The a-amylase family of enzymes has four subtypes: endoamylases, exoamylases, and transferases. The a-amylase subgroup hydrolyzes starch molecules at boiling and high temperatures. As a result, it creates maltose, glucose, and maltotriose.
AmyP is a raw-starch-degrading enzyme with potential applications in green fuel and food processing. It preferentially digests rice starch. AmyP has a specific activity of 118.5 + 0.6 U/mg at 40 degrees Celsius, which is higher than other raw-starch enzymes. AmyP hydrolyzes ten mg of rice starch in four hours. Similarly, it takes one hour to hydrolyze 80 mg of rice starch.
Amylase starts the starch digestion process by breaking down the long-chain saccharides into smaller pieces. Each starch chain contains two to three glucose units. Amylase is a member of the glycoside hydrolase family 13 and is produced in the pancreas and saliva.
a-Amylase is a fungal amylase
A-Amylase is a natural enzyme produced by the fungal genus Aspergillus. It is a fast hydrolase that is active in the neutral, acidic or mildly alkaline pH range. However, it is not as heat resistant as bacterial amylase.
A-Amylase has many applications in diagnostic and analytical chemistry. It can be used for the determination of fungus pathogens. Many species of Aspergillus and Rhizopus are used as sources of a-amylase.
A-Amylase is useful in fermentation and in commercial starch modification. It is also used in textile processing and cleaning compounds. In addition to these uses, it can also be used as a feed supplement to compensate for a deficiency in endogenous amylase in young animals. Fungal a-amylase hydrolyzes starch and turns it into fermentable sugars, most commonly maltose.
Amylases are enzymes that break down long chains of starch to form simple sugars. In particular, a-amylase converts amylopectin into maltose and maltotriose. Amylases are found in many plants and fungi.
NFAmy13A is a thermostable a-amylase that is active at a 60-degree temperature range. These properties make NFAmy13A a suitable candidate for use in the ethanol and food industries. It is also safe, which makes it an attractive candidate for a-amylase production in the biotechnological industry.
Amylases are a vital component of many industries. They can convert starch into sugar syrups and produce cyclodextrins for pharmaceutical applications. Moreover, they are cost-efficient and consistent and require less space and time for production. In addition, amylases are widely used in the food and bioprocessing industries.
a-Amylase inhibits starch recrystallization
A-Amylase is an enzyme that causes the degradation of starch molecules and reduces them to short-chain dextrins. It acts on the a-1,4 glycosidic bonds in starch polysaccharides and requires the presence of calcium ions for stability. Hence, it inhibits starch recrystallization by preventing the formation of double helices in starch.
A-Amylase is an enzyme that is present in our digestive system and breaks down carbohydrates from our diet. It catalyzes substrate hydrolysis through a double replacement mechanism involving the formation of covalent glycosyl-enzyme intermediates and oxocarbenium ion-like transition states.
The active site contains one carboxylic acid that acts as a nucleophile and a second carboxylic acid that is used as an acid/base catalyst and stabilizes the transition states during hydrolysis.
The presence of CBM and SBS on the a-amylase surface influences its starch-binding ability. Specifically, the aromatic residues are required for interactions with the partially negatively charged hydrogen atom of the substrate.
These aromatic residues must have a specific topology and conformations that are stable. While the presence of CBM and SBS does not affect catalytic activities towards short-chain polysaccharides, they are essential for the hydrolysis of long or insoluble starch.
Barley a-amylase is a 45 kDa enzyme that plays a vital role in starch degradation during germination. It releases sugars that provide energy to the plant embryo. It also contains a 19.6-kDa bifunctional protein called BASI. BASI inhibits both AMY2 and subtilisin family proteases, and it may protect the seed from pathogens secreting these proteases.
While a-amylase is useful for starch conversion, its use is limited by economics and the availability of high purity enzymes. However, some applications may require the use of a-amylase to improve the quality of products such as bread.
a-Amylase is a multifunctional enzyme
A-Amylase is an enzyme that has several independent functions. These enzymes are also called promiscuous enzymes or moonlighting enzymes. They are capable of catalyzing unrelated but complementary side reactions.
They are usually slow compared to their main activity and exhibit relaxed substrate specificity. These enzymes are beneficial to live organisms as they help them to survive and respond to changes in the environment.
Amy63 is a multifunctional enzyme produced by the marine bacterium Vibrio alginolyticus 63. Amy63 is an a-amylase with additional carrageenan and agarase activities. Amy63 shares seven conserved regions with typical a-amylases and clusters with similar enzymes from the GH13 family.
Amylases are multifunctional enzymes that break down starch into maltose and maltotriose. They are found in plants and fungi and are derived from various biological sources. Amylases are produced from different sources, including animal pancreas, yeast, wheat, and barley. Some are produced by large-scale fermentations of Bacillus and Aspergillus species.
Amylases are metalloenzymes and require a metal cation as a cofactor. Calcium is one such cofactor. Different enzymes have different requirements and this needs to be taken into account. Analytical scales with a sensitivity of 0.0001 g are usually required for measurements. Analytical scales with lower sensitivity are also available.
Although most studies on the a-amylase enzyme are conducted on a laboratory scale, there is no guarantee that the enzyme will perform in any given situation. The best way to ensure that a-amylase is as safe and effective as possible is to ensure its purity.
A-Amylase enzymes are used in a variety of applications, including conservation. However, they have several disadvantages. Often, their concentrations are too high and cause them to become inactive. Additionally, the enzymes are inactivated by hot water or ethanol, which disrupts their tertiary structure and terminates their hydrolytic activity. This can leave residues on the artifact.
Additionals: